2015 was a busy year in the digital health devices industry. There were many advances in apps and devices designed to improve telemedicine and patient engagement, wearables monitoring chronic diseases, digital devices to assist visual impairment and rapid diagnostics for remote healthcare. This progress was assisted by continued investor interest. A recent year end review by Rock Health reported that in 2015 digital health venture funding topped $4.3B, maintaining a steady 7% of total venture funding while other sectors dipped.
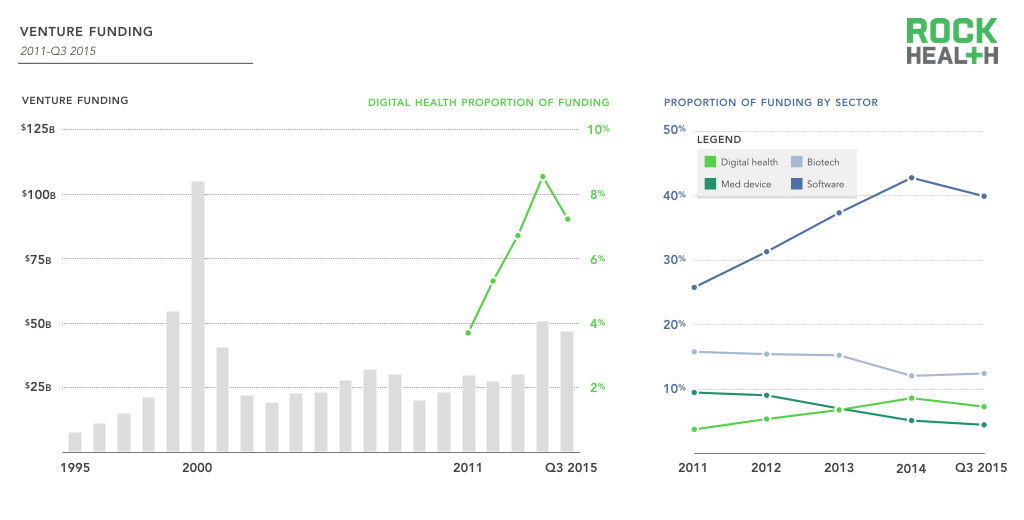
Source: PwC MoneyTree (latest available is through Q3 only); digital health data based on Rock Health data. Note: Digital health only includes U.S. deals >$2M
The prospects for continued advances in 2016 look good. A new wave of digital health innovation is expected, powered by the increasing capability to stream and analyze data.
Here are a few of my favorite 2015 digital health devices:
Telemedicine
Does your child get frequent ear infections? No problem,
ear exams can now be performed at home and streamed to a physician for diagnosis. CellScope
introduced an ear probe attachment (Oto) that clips over an iPhone camera. The
device streams footage of the inside of the ear to an app which can then be
analyzed by a doctor on call.
United Healthcare announced it would begin coverage of virtual doctor visits through Doctor On Demand, AmWell and NowClinic.
Wearable Monitoring Devices
for Patients
Biotech Tattoos created buzz with their ability to monitor vital signs through electrical components worn directly on the skin. San Diego's UCSD nanoengineering department plans to take bio tats a step further by combining "bio ink" with a wearable sensor resulting in temporary tattoos that measure glucose levels in diabetics.
Google secured a patent for their prototype contact lens
and reader/display unit to monitor glucose levels in diabetic patients.
MIT announced development of a prototype “smart” bandage to monitor wound healing, administer medication and alert a physician if further medical care is needed.
Digital Devices for the Visually Impaired
Be My Eyes smartphone app helps visually impaired people navigate everyday life through the assistance of sighted volunteers.
MIT announced they had completed a prototype for the Finger Reader, a wearable that reads text in real time. The device connects to an Android phone or tablet. Development of a wireless version that works with iPhones is in progress.
Patient Engagement
Minnesota Healthsolutions Corporation, Mayo and Novu received NIH funding to perform a study of the effects of digital health on COPD patient care.
Rapid Diagnostics for Remote Healthcare
Diagnostics for All foundation and Harvard University developed a prototype $2 paper-based microfluidic DNA rapid diagnostic device that can be used anywhere with the aid of a smartphone to read results.
Diagnostics for All foundation and Harvard University developed a prototype $2 paper-based microfluidic DNA rapid diagnostic device that can be used anywhere with the aid of a smartphone to read results.






